Why oceans keep absorbing our carbon dioxide
The ocean plays a major role in reducing the impact of the changing climate. About a quarter of the carbon dioxide (CO2) released due to human activities has been taken up by ocean water. However, this uptake is not without consequences for ocean organisms and the different biogeochemical processes taking place in the ocean. Ocean alkalinity, often described as the excess of bases, is one of the main ocean properties that governs the efficiency of carbon dioxide uptake.
An important aspect of ocean alkalinity is its ability to provide resistance to changes in pH. Because of ocean alkalinity, ocean water is able to buffer against acidic inputs, like those from the uptake of carbon dioxide. In a new review paper, written by NESSC-researcher Jack Middelburg (Utrecht University), Karline Soetaert (NIOZ) and Mathilde Hagens (Wageningen University), various aspects of ocean alkalinity and the role of the ocean in carbon cycling during times of global change, are discussed in depth. The new paper, published online in Review of Geophysics, revisits old views and proposes new ones.
Different processes
Ocean alkalinity is critical to the uptake of CO2, but is impacted by a wide range of different processes, both on short and long time scales, the authors write. For example, the addition of a strong acid to the ocean, such as acid rain, decreases alkalinity, whereas an addition of lime increases alkalinity. When organic matter is broken down in the water column, alkalinity is decreased, but degradation processes without oxygen (by and large) increase alkalinity. Also the formation and dissolution of minerals in shells and corals affect alkalinity.
On even longer time scales (tens of thousands of years), ocean alkalinity is governed by the weathering of minerals on land, eventually entering the ocean, and the formation of calcium minerals and shells by organisms, like corals. The many sources that contribute to ocean alkalinity makes it difficult to estimate the total budget of ocean alkalinity, and the authors urge for more research on several possible inputs, like the influx of inorganic carbon in particles from rivers.

Warning sign
Importantly, the increasing amounts of carbon dioxide in the earth’s atmosphere also impacts oceans’ carbon storage. Carbon dioxide taken up by the ocean affects the chemistry of seawater, a process known as ocean acidification. It also reduces the capacity of the ocean to further remove carbon dioxide from the atmosphere.
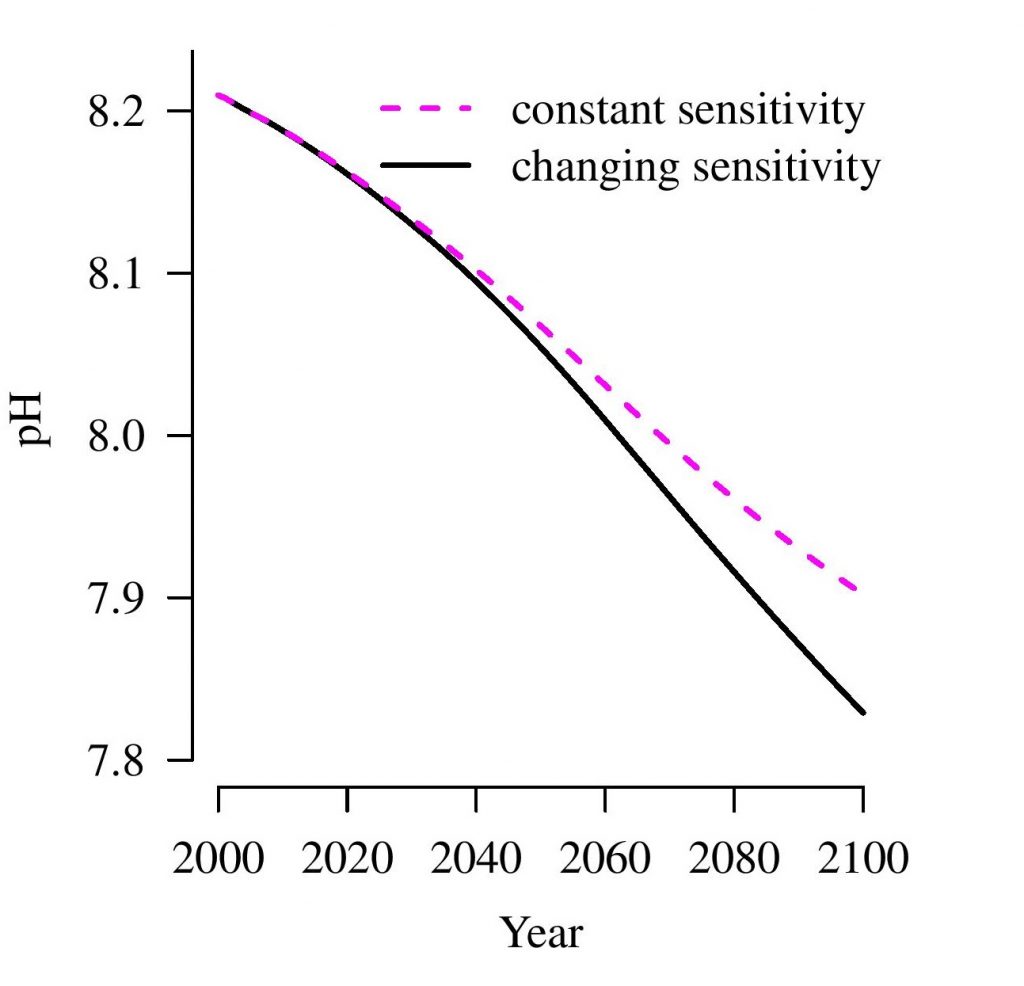
The article further cautions that, although changes in alkalinity will be moderate for centuries to come, current measurements show systematic changes in ocean alkalinity. These measurements serve as early warning signs that acidification of ocean water is reaching greater depths. It is estimated that by the end of this century, the pH of ocean water could already drop significantly.
Confusion
The concept of alkalinity is still associated with confusion, the article notes, because it is used in different disciplines using different definitions. The new paper resolves the subtle differences in definitions and indices used in different research fields when, for example between marine and freshwater scientists and between chemists and geologists. These differences become important when studying biogeochemical processes, such as mineral precipitation. The authors have clarified the usage of commonly applied terms to prevent further confusion in the future.
Article:
Ocean Alkalinity, Buffering and Biogeochemical Processes
Reviews of Geophysics, 2020
Middelburg, J. J., Soetaert, K. and Hagens, M.
https://doi.org/10.1029/2019RG000681

