Cells of cable bacteria sacrifice themselves
Cells of electricity-generating cable bacteria sacrifice themselves for the greater good of the organism, a new NESSC-publication shows. The researchers discovered that electricity-generating cable bacteria do not divide labour equally. Where some cells generate energy for the ‘cable’ to survive, other cells cannot generate enough energy, and sacrifice themselves for the greater good of the organism. This strange ‘electrical community service’ is a new discovery in biology, not observed before in any other multicellular organism.

“This forces us to rethink our understanding of multicellularity,” says PhD student Nicole Geerlings, the lead author of the study. Researchers from Utrecht University, among others NESSC-researchers Jack Middelburg and Lubos Polerecky, University of Antwerp and Delft University of Technology discovered that the division of labour in cable bacteria or cable cells has another dimension.
“Usually each cell generates its own energy, even in complex multicellular organisms like humans,” explains Prof. Filip Meysman from the University of Antwerp. Electrons are stripped from an energy-rich electron donor and transported along the cell membrane to an electron acceptor. This generates the energy that a cell needs to grow and reproduce. Cable bacteria have evolved an ingenious way to generate energy by dividing the tasks between cells within their chains.
Electricity generating bacteria
Cable bacteria are centimetre-long micro-organisms that consist of thousands cells stacked on top of each other in a chain called a filament. These multicellular bacteria, only discovered a few years ago, act like electrical cables. Hence the name ‘cable bacteria’. They can conduct electricity over long distances up to several centimetres, using sulphide or oxygen as an electron acceptor. The filament gets its energy when electrons are sent through the cable from a molecule in one location to another molecule elsewhere.
Cells in the deeper layers of the seafloor strip high-energy electrons from sulphide, these electrons are then transported through internal electrical cables in the filament to the top of the seafloor where other cells give the electrons to oxygen. This means that the cells within a cable are dependent on each other and have to work together to get energy needed for growth and reproduction. This ‘electric metabolism’ gives cable bacteria a huge advantage, since they can get energy from the deeper layers of the seafloor.
Now, results from the new study show that energy generated by the electric metabolism is used unevenly between the cells: only the cells that use sulphide get enough energy to take up nutrients and grow. Cells that use oxygen cannot grow, and sacrifice themselves for the greater good of the rest of the organism.
Oxygen needed
Cable bacteria are on the one hand dependent on oxygen because the filament needs to get rid of the electrons. “Cable bacteria can give away a lot of electrons to oxygen very fast. They can be responsible for a large fraction of oxygen usage in the seafloor,” explains Dr. Cheryl Karman, who performed electrochemical analysis on the cable bacteria. The presence of oxygen is essential for the survival of the filament. If cable bacteria are not connected to oxygen, they stop transporting electrons and none of the cells get energy.
On the other hand, oxygen is also harmful to cells, and long exposure to oxygen would cause these cells to die. Because of this, the cells reacting with oxygen provide a sort of ‘community service’ to the rest of the filament. These cells help the rest of the cells in the filament, but they are at a disadvantage because they cannot generate energy and could potentially die.
“Surprisingly, all cells in a filament always have the machinery present to give electrons to oxygen,” explains Meysman. Experimental data showed that when the outside environment of cells is changed from sulphidic to oxic, the cells immediately started to give electrons to oxygen. They can switch roles when the outside environment changes. Each cell can do both reactions, none of the cells is specialized.
At the moment, it is unknown if cells are able to carry out this ‘community service’ until they die and are then replaced by new cells, or if cells within a filament switch roles and each cell only has to do ‘temporary community service’.
“We thought something went wrong”
The uneven growth between the different cells was discovered by feeding the cable bacteria labelled nutrients and then measure the uptake of these nutrients with an ion imaging technique called nanoSIMS. “The nanoSIMS allows us to track which cells have taken up the labelled nutrients and were therefore growing,” explains dr. Lubos Polerecky, assistant professor in nanoSIMS analysis from Utrecht University.
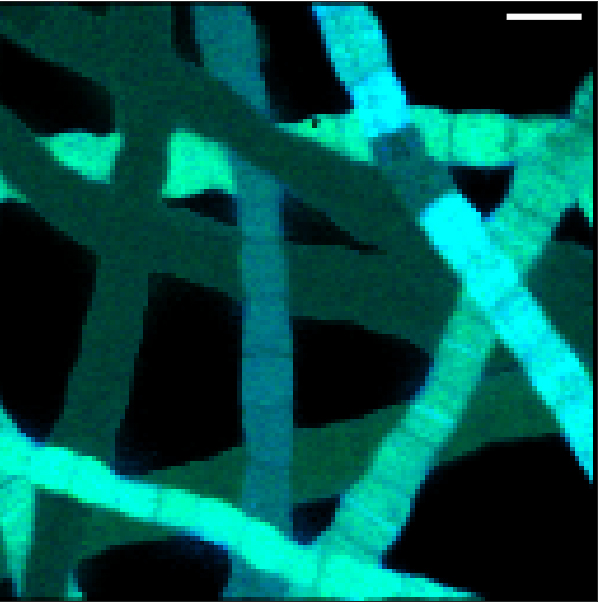
“When we first observed that there was no nutrient uptake in the cells that were in contact with oxygen we thought something went wrong with the experiment. We did it again, with the same results,” Geerlings explains.
In contrast, the cells that had access to sulphide took up a lot of nutrients and were growing fast, generating enough energy for growth and reproduction. This lead to the conclusion that only the cells in contact with sulphide could generate enough energy for growth and the cells with access to oxygen simply transferred electrons without energy generation.
Publication:
Division of labour and growth during electrical cooperation in multicellular cable bacteria
Proceedings of National Academy of Sciences, 2020.
N.M.J. Geerlings, C. Karman, S. Trashin, K.S. As, M.V.M. Kienhuis, S. Hidalgo-Martinez, D. Vasquez-Cardenaz, H.T.S. Boschker, K. De Wael, J.J. Middelburg, L. Polerecky, F.J.R. Meysman.
doi: 10.1073/pnas.1916244117

